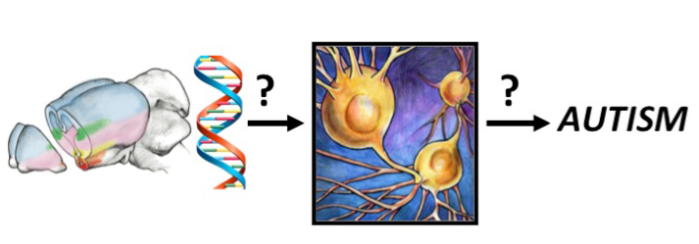
Project 1. Uncovering the developmental genetic programs for establishment of Amygdala neuronal diversity and connectivity.
Proper displays of social interaction between members of the same species are required for species propagation and survival. These behaviors are regulated in large part via the amygdala, a central limbic system structure highly conserved across mammals. Alterations in specific aspects of amygdala development are a hallmark feature of human disorders such as autism and schizophrenia.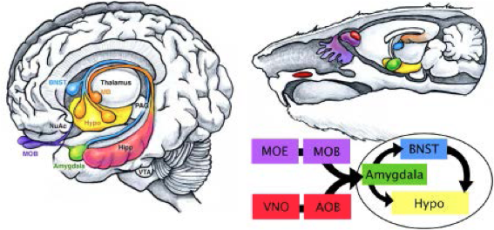 While environmental inputs shape social behaviors, many components of these behaviors are hard-wired. Thus, it is likely that developmental genetic programs pattern the wiring of brain circuits that control these behaviors. However, the genetic programs that drive formation of amygdala neuronal identity and circuitry remain largely unknown. We are currently addressing this question using a combination of developmental genetic, gene profiling, electrophysiological and behavioral approaches.
While environmental inputs shape social behaviors, many components of these behaviors are hard-wired. Thus, it is likely that developmental genetic programs pattern the wiring of brain circuits that control these behaviors. However, the genetic programs that drive formation of amygdala neuronal identity and circuitry remain largely unknown. We are currently addressing this question using a combination of developmental genetic, gene profiling, electrophysiological and behavioral approaches.
Project 2. Determination of how developmentally defined limbic sub-circuits control sex-specific innate social behaviors.
Despite a general understanding of how different brain structures control innate behaviors, the sub-circuit logic for regulation of different innate behaviors remains generally unknown. Moreover, many of these behaviors differ between sexes suggesting different patterns of connectivity and/or circuit function in males and females.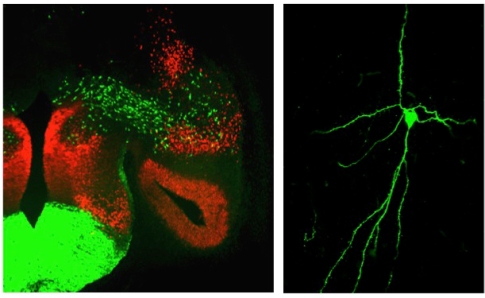 From our studies, we have discovered that parcellation of amygdala progenitor pools by transcription factor expression predicts neuronal subtype identity as defined by molecular and intrinsic electrophysiological characteristics, and quite interestingly sex differences in innate behavior-tuning specificity. Using a combination of viral, electrophysiological and optogenetic approaches our goal is to generate a comprehensive limbic system neuronal circuit map based on developmental genetic criteria. Moreover, we are employing a combination of in vivo optogenetic and pharmacological approaches to define the necessity and sufficiency of developmentally defined limbic sub-circuits in regulation of sex-specific innate behaviors.
From our studies, we have discovered that parcellation of amygdala progenitor pools by transcription factor expression predicts neuronal subtype identity as defined by molecular and intrinsic electrophysiological characteristics, and quite interestingly sex differences in innate behavior-tuning specificity. Using a combination of viral, electrophysiological and optogenetic approaches our goal is to generate a comprehensive limbic system neuronal circuit map based on developmental genetic criteria. Moreover, we are employing a combination of in vivo optogenetic and pharmacological approaches to define the necessity and sufficiency of developmentally defined limbic sub-circuits in regulation of sex-specific innate behaviors.
Project 3. Translation of our knowledge of limbic system development toward understanding genotype to phenotype links in autism spectrum disorders.
Currently, autism diagnosis is solely based on behavioral criteria. This lack of biological criteria for diagnosis represents a severe limitation for both the design of rational therapeutic approaches to treat underlying neurological dysfunction and predict outcomes for individuals with autism. Thus, there is a pressing unmet need to understand how mutations in autism susceptibility genes relate to neuroatypical behavior that defines autism. To address this question, focusing on genes that have been implicated in amygdala development, we are linking autism gene function to altered trajectories of amygdala development.